- Home
- Biological Products
- Mesenchymal Stem Cells
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are a type of stem cells that process the ability of self-renewal and multipotency, which could differentiate into cells of many germ layers, including adipocytes (fat), osteocytes (bone), chondrocytes (cartilage), hepatocytes (blood), neurons ( nerve), muscle cells, epithelial cells (skin), pancreatic cells, etc. Moreover, evidence indicates that MSCs possess potential immune boosting, anti-inflammatory and nutritive properties.
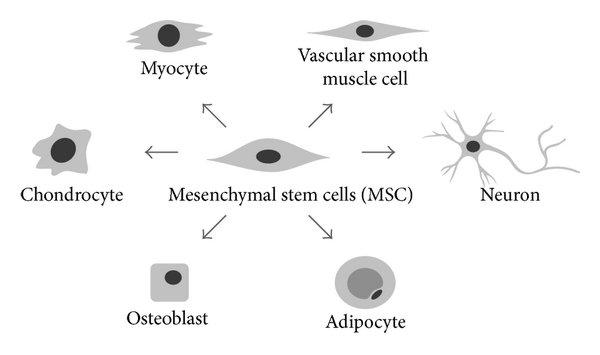
MSCs have several advantages, such as easy availability as well as few ethical concerns.
MSCs are showing great potential in the fields of regenerative medicine, tissue engineering and gene therapy. They can differentiate into a variety of cell types, including bone, cartilage, muscle, nerve and many others. Scientists have developed proven methods for the expansion of cord tissue stem cells, allowing a potentially unlimited supply of this powerful resource.
What conditions are best treated by MSCs?
With over 3000 clinical trials and numerous research studies in process, scientists predict an explosion of new therapies being universally adopted, including standard, routine treatments for diabetes, multiple sclerosis, stroke, heart failure and liver damage.
In the field of regenerative medicine, MSCs can improve the conditions of renal failure, cirrhosis, fertility, osteoporosis, and ischemic heart disease.
MSCs from the Wharton Jelly
Wharton's jelly is the gelatinous substance within the umbilical cord that surrounds the umbilical vessels. It protects and insulates umbilical blood vessels during pregnancy. Upon delivery, umbilical cord is routinely separated with the placenta and sends for discard. Scientists have found that your baby Wharton's jelly is an exceptional source of valuable stem cells.
MSCs are Proven Safe
In 2017, more than ten publications used expanded MSCs in both local injection and infusion. All publications showed expanded MSC transplantation was safe. Some studies followed-up 12 months, others followed up to 6 years (Bertolucci Jorge, et.al. Circulation Research. 2017-SEP)
Lumbar degenerative disc disease-thirty-three patients injected with their expanded MSCs and followed up to 6 years post-treatment. There was no severe side effects. (Centeno Christopher et.al. Journal of Translational Medicine. 2017-Sep)
12 chronic stroke patients were intravenously infused with autologous expanded MSCs. All patients were followed up the 208th week without any cell related side effects. (Bhasin Ashu et.al. Cerebrovascular Diseases Extra. 2011)

Quality Control Program
Our Quality Control Program (QCP) to detect microbiological contamination is in compliance with the American Association of Blood Banks and European Pharmacopoeia:
Sterility test
Gram stain
Detection of mycoplasma
Endotoxin assay
Hepatitis B
Hepatitis C
Cytomegalovirus
HIV
VDRL Test -Syphilis
HTLV- Human T-lymphotropic virus
CBC
